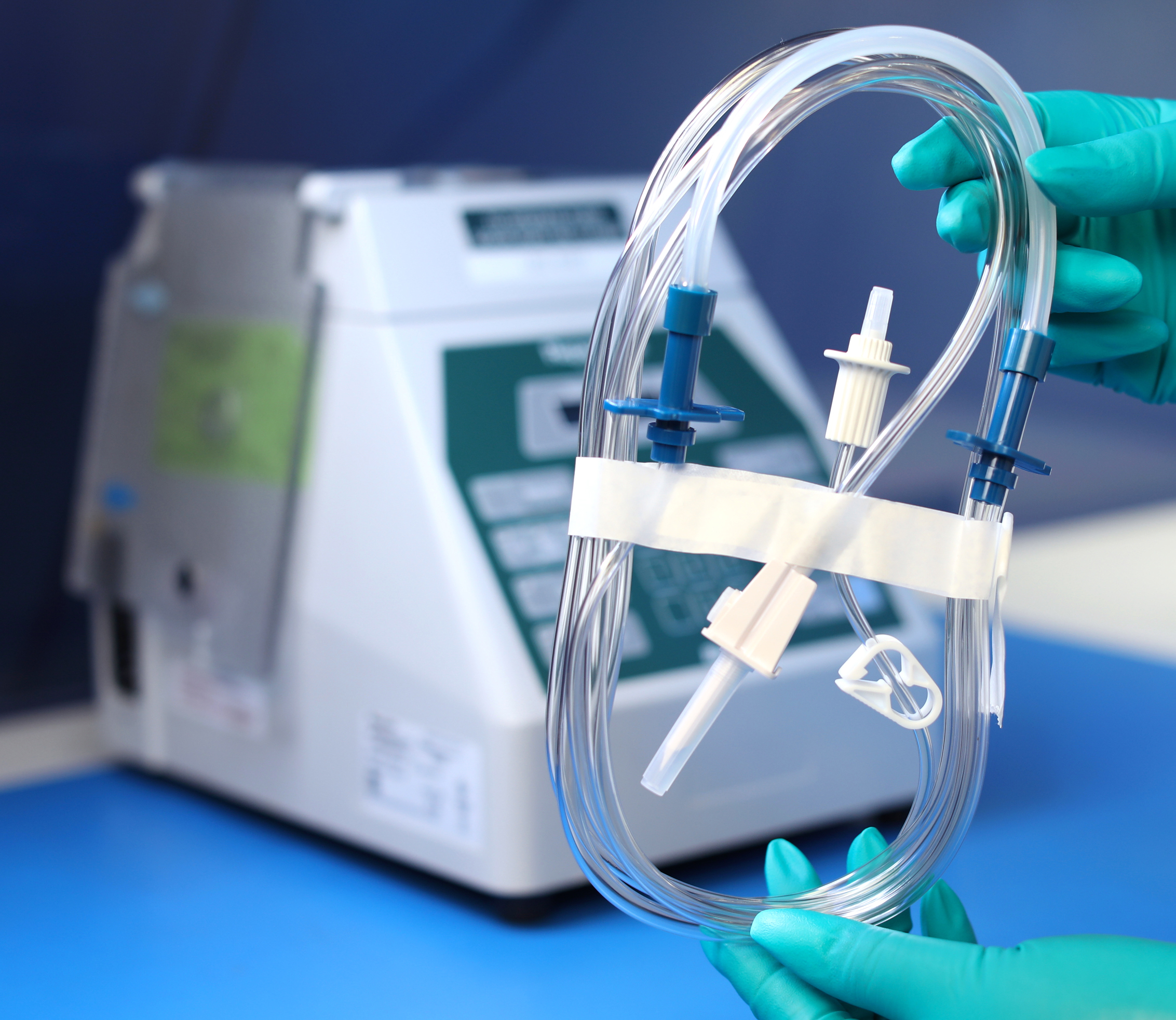
Product Offerings
MPS Pharma Baxter REPEATER™ Pharmacy Pump Compatible Tube Sets:
Rigorously tested to perform in parity with official Baxter products.
- Cost Effective Alternative to Baxter Pump Tubing Sets
- Male Luer Lock with Contoured Finger Grip
- 0.2µm Micron Vent Filter on spike ensures the quality of air entering the solution line during venting
- Non-DEHP Tubing
Dimensions, Features, and Performance that closely match
Baxter REPEATER™ Pharmacy Pump Sets
Physical Comparison Data
See our detailed Baxter Equivalent Tubing Physical Characteristics & Performance Comparison for more details.
The Upgraded MPS 0.2 um Spike
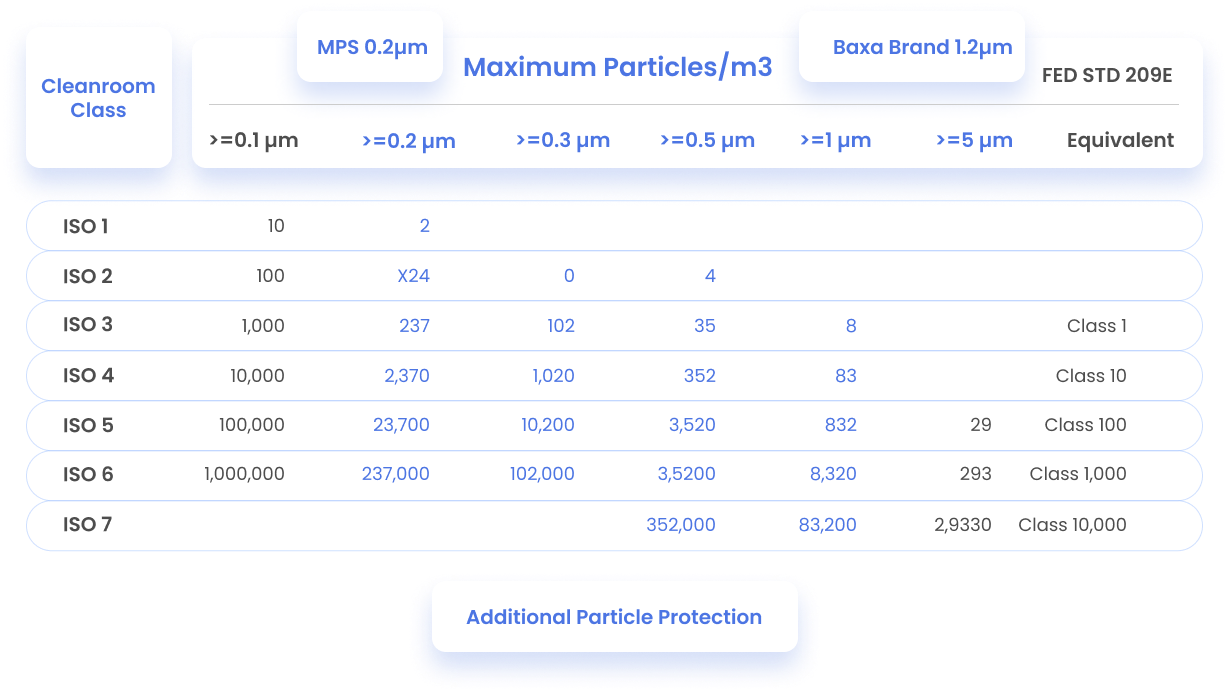
Frequently Asked Questions
What final release testing is performed for these products?
| Test | Method |
| Sterility | Direct Transfer Method Embedded Spore Strip accredited to the ISO 17025 |
| Endotoxin/LAL | ISO 17025 – (USP), General Chapter <85>, (USP), General Chapter <161>, Medical Devices |
All testing is performed by qualified third party testing labs contracted with MPS Pharma. The final measurements are all reported on our current Certificate of Analysis.
Can I see a sample Certificate of Analysis that comes with this product?
See the attached PDF for our current Certificate of Analysis
Can I get a specification sheet of this item for my records?
See the attached document for the specification sheet
Do you have a comparative document of your set versus the original Baxter Brand?
See the attached document for comparison
Our pharmacy follows USP <797> and it requires we use depyrogenated materials. Are your products depyrogenated? Do you have a Certificate of Analysis (C of A) stating such?
All MPS lots are tested for endotoxins (pyrogens) and will meet the materials requirements as defined in USP <797>. A detailed explanation of this can be found in this PDF link. All MPS C of As include measurements for these endotoxins.
Have any questions?
Contact our Customer Service team for additional support at
(844) 641-3814 or by email at sales@mpspharma-inc.com

 0
0