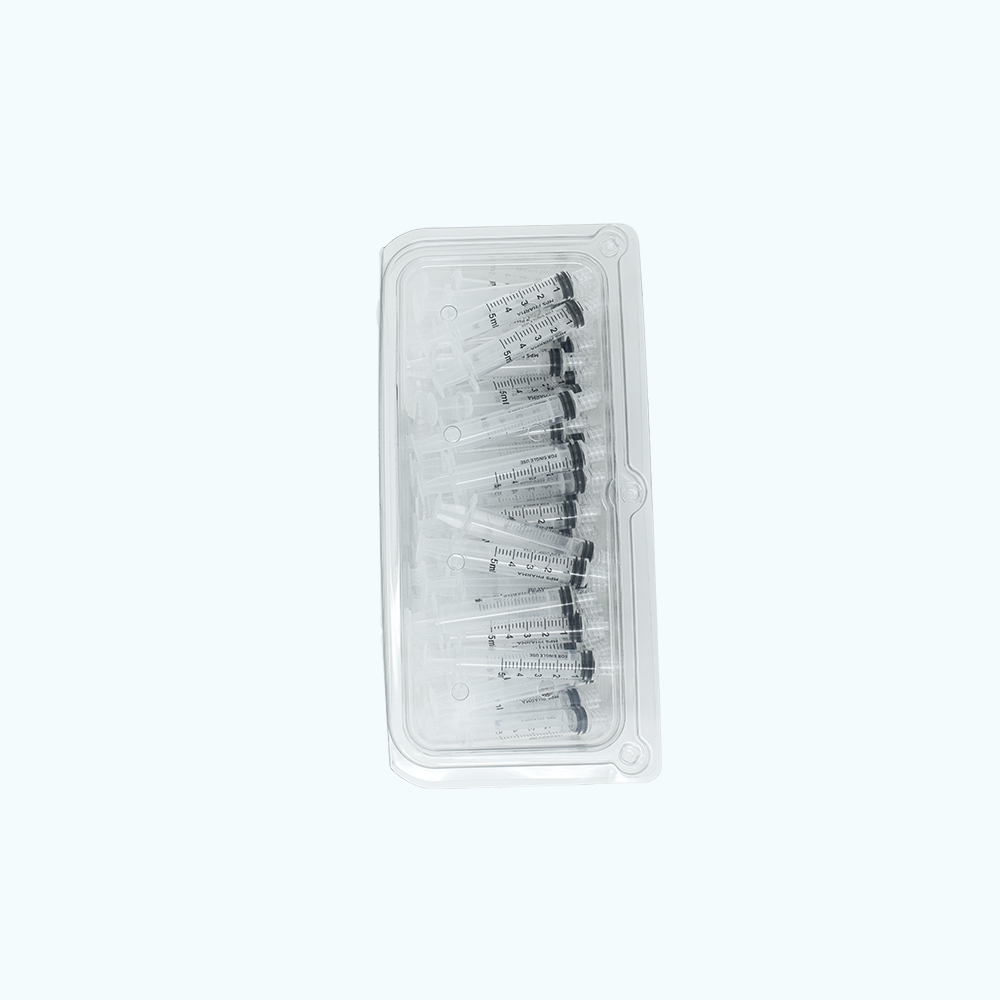
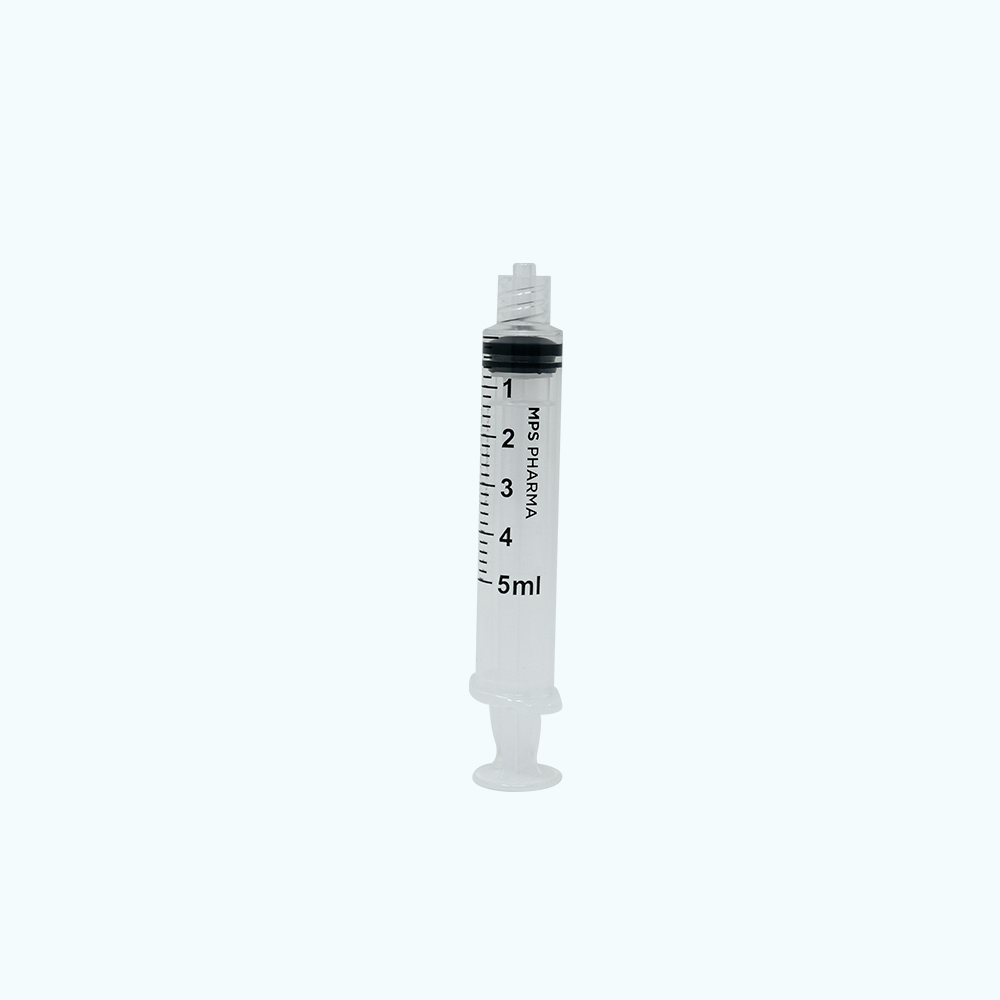
Sterile Syringe Convenience Trays – 5 mL, Luer Lock
50 pack 5 mL MPS Pharma Luer Lock Syringes, Sterile. Double Pouched and Placed into Tray. 12 Trays of 50 Syringes Per Box.
| Manufacturer Equivalents | |
|---|---|
| Becton Dickinson | 309703 |
| Cardinal Health Covidien Monoject | 8881516200 |
5 mL size sterile syringe convenience trays designed directly with input from compounding pharmacists and technicians to create the ‘Best in Class’ option available to compounders. Each pack contains 50 - 5 mL syringes per tray. Each box contains 12 trays for a total of 600 syringes per box. These sterile syringe convenience trays can be utilized by pharmacy staff for the preparation of compounded injectable medications.
Sample products are available to pharmacies seeking to validate these syringes as a container closure system for their drugs. These sterile syringes can be utilized as a container closure system to safely dispense aseptically filled compounded injectable grade medications or approved injectable drug products to the full spectrum of patients.
Features:
- Single-direction orientation of syringe tips to assist in batch preparation
- Larger pack size reduces number of handling steps helping staff save time over other tray options.
- Double-layer packaging. Each tray is wrapped with two sterile barrier pouches. Easily support compliance with USP 797 wipe down requirements from warehouse storage to Class 7 space to final Class 5 filling space.
- Each pouch and tray are labeled with identifying information. Full lot traceability of material through each USP 797 compliant wipe down step and use inside filling hood.
- Total particulates are sampled, measured, and released to method tracible to USP <788>. Ensure each compounded batch is compliant to the injectable drugs requirements.
- Final release testing includes sterility, endotoxin, and particulate testing to USP <788>. All results are reflected on a lot number specific Certificate of Analysis
- Sizes available from MPS include 3 mL, 5 mL, 10 mL, 20 mL, 30 mL, and 60 mL
- Private branding options – Ability to print your company logo on barrel of syringe.
- Supply agreement options available. Ensure your pharmacy never runs low on supply by partnering with a compounding pharmacy focused company.
More detailed product information for this item is available through our FAQ page.
What final release testing is performed for these sterile syringe convenience trays?
| Test | Method |
| Sterility | Direct Transfer Method Embedded Spore Strip accredited to the ISO 17025 |
| Endotoxin/LAL | ISO 17025 – (USP), General Chapter <85>, (USP), General Chapter <161>, Medical Devices |
| USP <788> Test Method 1 – Particulates | Test Method Acceptance Criteria: Based on the methodology of USP <788> |
All testing is performed by qualified third party testing labs contracted with MPS Pharma. The final measurements are all reported on our current Certificate of Analysis.
Why do you measure particulates to USP <788>?
Our syringes are most used as a delivery system for injectable preparations. USP <788> Particulate Matter in Injections was specifically written to address this type of usage. The chapter provides compounders with allowable limitations of particulate matter in form of undissolved particles, other than gas bubbles, unintentionally present in the solutions.
USP <788> is applicable to finished drug products; empty syringes themselves are not considered finished drug product. The testing MPS Pharma has performed on the syringes is based on the methodology of USP <788>, with particulate measurements reported for 10 um and 25 um sizes against limits reported on a per container basis.
All particulate measurements are reported on per lot basis and a final number is provided in our Certificate of Analysis.
Can I see a sample Certificate of Analysis that comes with this product?
See the attached PDF for our current Certificate of Analysis
What are the syringes made of? Can more information be shared?
The syringe barrel and plunger are composed of polypropylene. The stopper is composed of polyisoprene. These are standard materials used across syringe manufacturers.
A more detailed material profile is available to share with customers who complete a non-disclosure agreement.
Why use two layers of packaging for your convenience trays?
Traditional syringe convenience packaging offered by the common core manufacturers are typically just a single sterile barrier consisting of a single tray with a lid. And the tray may only hold up to 25 units.
Our trays are covered by two sterile layers. The layers can be removed between material transfer steps before reaching the final filling space. (Example from warehouse space to ISO 7 space, and from ISO 7 space to ISO 5 filling area). The larger pack sizes (up to 60 syringes a pack) allows for less material handling and wipe down steps. These layers offer compounding operations improved control over material flow, less handling, and cut down on overall labor waste made by the traditional packaging.
Are dimensional drawings of the syringes available?
Detailed dimensional drawings are available to customers.
Can I get a specification sheet of this item for my records?
See the attached document for the specification sheet
Can I get our company logo added to the syringe?
MPS can print your company’s logo on the syringe. It can be printed at the base beneath the numbers towards the plunger or along the barrel. The same syringe can be placed into our tray packaging and supplied to your pharmacy. The set-up involves a one-time plate charge for each size syringe. All existing quality release criteria will still apply.
Our pharmacy follows USP <797> and it requires we use depyrogenated materials. Are your products depyrogenated? Do you have a Certificate of Analysis (C of A) stating such?
All MPS lots are tested for endotoxins (pyrogens) and will meet the materials requirements as defined in USP <797>. A detailed explanation of this can be found in this PDF link. All MPS C of As include measurements for these endotoxins.

 0
0