Product Offerings
-
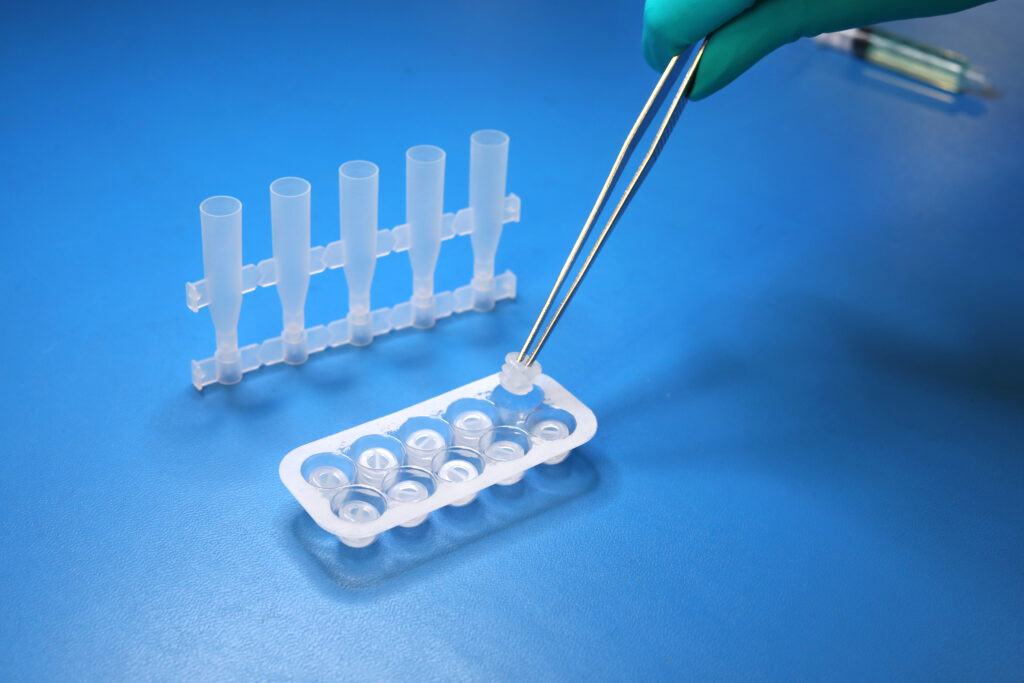
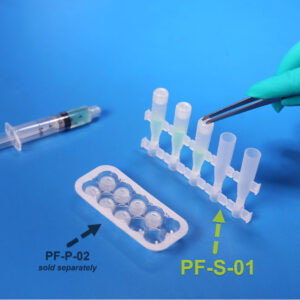 PF-S-01
PF-S-01SafeDate™ Low Volume BUD Compliant Ophthalmic Dropper Bottle
Catalog #: PF-S-01 Quantity per Box: 1000Prime Volume (mL): 1.5 mLProduct Features: Bottle, Natural, Preservative Free, SafeDate -
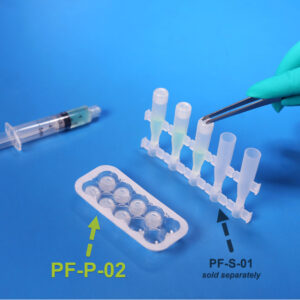 PF-P-02
PF-P-02SafeDate™ Low Volume BUD Compliant Ophthalmic Dropper Cap
Catalog #: PF-P-02 Quantity per Box: 1000Product Features: Cap, Natural, Preservative Free, SafeDate
Frequently Asked Questions
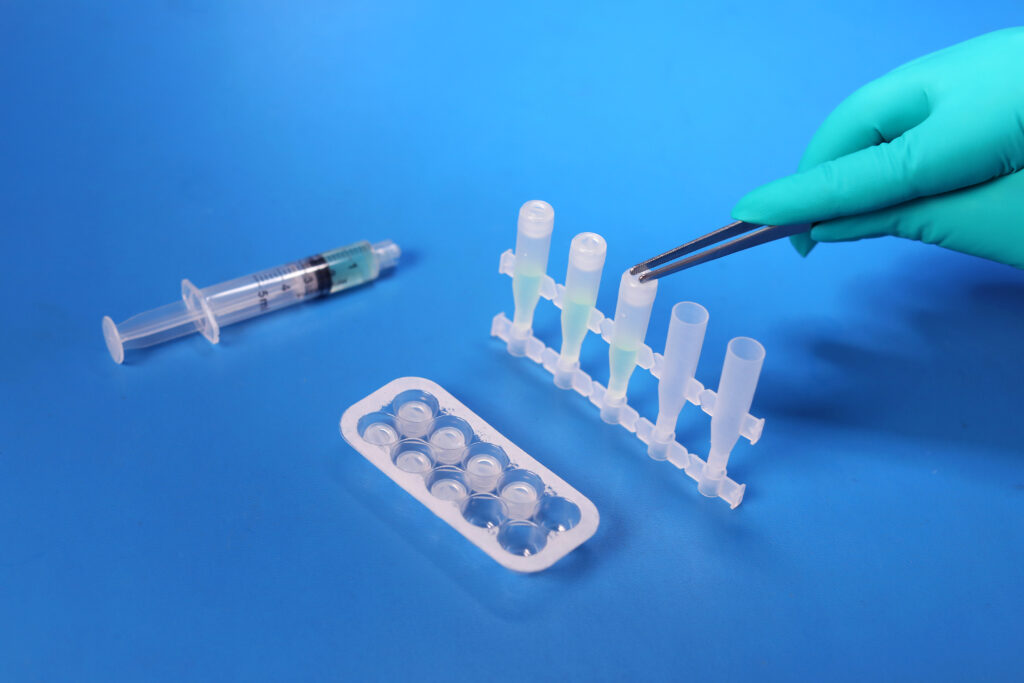
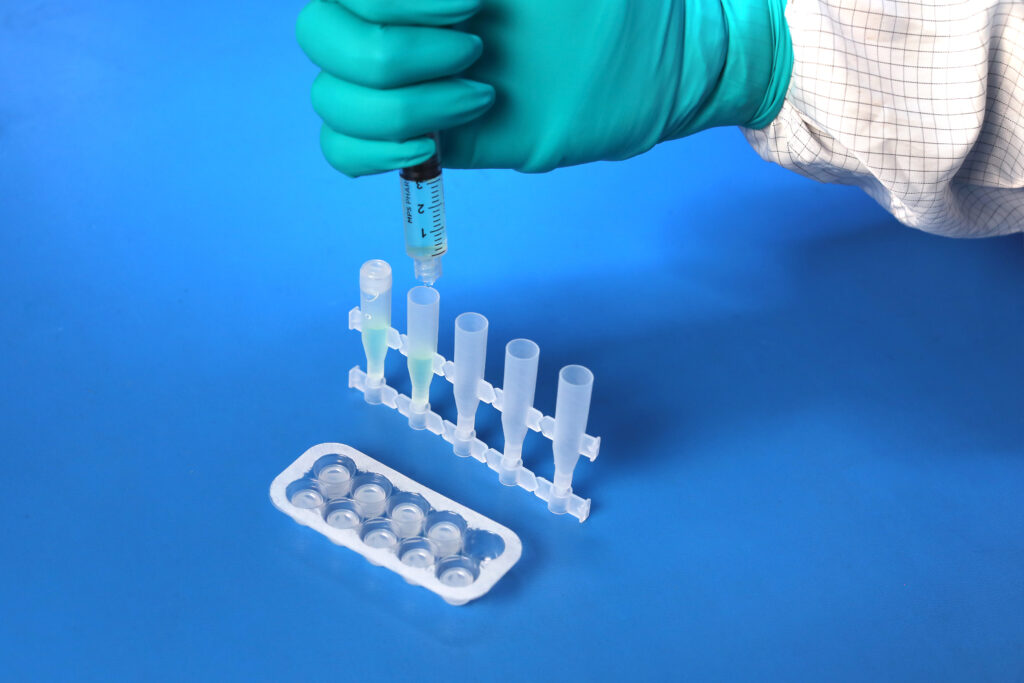
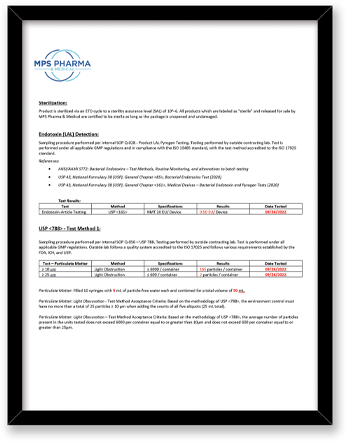
What final release testing is performed for these bottle products?
|
Test |
Method |
Component |
|
Sterility |
Direct Transfer Method Embedded Spore Strip accredited to the ISO 17025 |
Bottle, Cap |
|
Endotoxin/LAL |
ISO 17025 – (USP), General Chapter <85>, (USP), General Chapter <161>, Medical Devices |
Bottle, Cap |
|
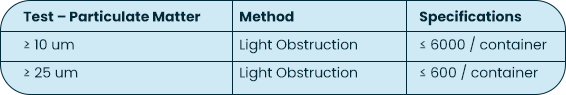
USP <788> Test Method 1 – Particulates |
Test Method Acceptance Criteria: Based on the methodology of USP <788> |
Bottle |
All testing is performed by qualified third party testing labs contracted with MPS Pharma. The final measurements are all reported on our current Certificate of Analysis.
Why do you measure particulates to USP <788>?
Our bottles are most used for topical ophthalmic preparations in the form of eye drops. This is a form of extraocular application of medication to the eyes. Specific chapters in USP guidelines state as follows:
USP <771> Opthalmic Products – Quality Tests:
“Two general categories apply for product administration to the tissues in the eye. Intraocular administration includes all ophthalmic products that cross (penetrate) boundary tissue, such as the cornea and sclera. For subvisible content, USP guidance is followed. Products for intraocular use must comply with Particulate Matter in Ophthalmic Solutions <789>. Product for extraocular use must comply with Particulate Matter in Injections <788>.”
Both USP <788> and <789> are applicable to finished drug products; containers are not considered finished drug product. The testing MPS Pharma has performed on the bottles is based on the methodology of USP <788>, with particulate measurements reported for 10 um and 25 um sizes against limits reported on a per container basis.
Can I see a sample Certificate of Analysis that comes with this product?
If I open the pouch, will the bottle contents still be sterile?
Outside the bottle:
Once the pouch has been opened, then by the definition the outside of the bottle cannot be considered sterile, unless the pouch is opened in a controlled sterile environment.
Inside the bottle:
- Inside of the bottle of an opened pouch is sterile and will remain sterile if pouches are opened in a clean and sterile environment. To maintain sterility, pouches should be handled and closed in the same controlled environment.
- MPS does not own any specific drugs of its own, and as a result has performed the CCIT test with water. MPS bottles pass the Container Closure Integrity Testing (CCIT) as defined by USP <1207>.
- Many clients of MPS have performed CCIT protocols against the bottle combined with their specific drugs and they have successfully passed.
- CCIT is not a sterility test. The CCIT methodology evaluates the container’s ability to maintain a container closure system in protecting the internal contents against potential external contamination.
The passing of these tests provides additional confidence that no external contaminants can enter the closure system.
What is the drop size for these bottles?
Drop size is dependent on several factors including the product being dispensed and the technique in which it is dispensed. The best advice for customers is to test the dropper tip with the compounded product to determine its suitability in its application.
A general water reference point can be provided to our customers as a starting guideline:
| Size(s) | Drop Size |
| 1 mL | 42 ul +/- 5 uL |
What are the bottles made of? Can more information be shared?
The bottle is composed of low-density polyethylene resin (LDPE). The cap itself is composed of polypropylene (PP).
Can I get a specification sheet of this item for my records?
See the attached document for the specification sheet
Our pharmacy follows USP <797> and it requires we use depyrogenated materials. Are your products depyrogenated? Do you have a Certificate of Analysis (C of A) stating such?
All MPS lots are tested for endotoxins (pyrogens) and will meet the materials requirements as defined in USP <797>. A detailed explanation of this can be found in this PDF link. All MPS C of As include measurements for these endotoxins.
Have any questions?
Contact our Customer Service team for additional support at
(844) 641-3814 or by email at sales@mpspharma-inc.com

 0
0