Product Offerings
-
 UA15-415-10-BOT-10
UA15-415-10-BOT-1010ML Sterile Dropper Bottle, Natural
Catalog #: UA15-415-10-BOT-10 Quantity per Box: 100Product Features: 10 mL, Bottle, Custom Dropper, UA15-415 -
 UA15-415-10-BOT-10-WHT
UA15-415-10-BOT-10-WHT10ML Sterile Dropper Bottle, White
Catalog #: UA15-415-10-BOT-10-WHT Quantity per Box: 100Product Features: 10 mL, Bottle, Custom Dropper, Light sensitive, UA15-415 -
 UA15-415-10-BOT-15BR
UA15-415-10-BOT-15BR15ML Sterile Dropper Bottle, Natural, Boston
Catalog #: UA15-415-10-BOT-15BR Quantity per Box: 100Product Features: 15 mL, Bottle, Custom Dropper, UA15-415 -
 UA15-415-10-BOT-15CR
UA15-415-10-BOT-15CR15ML Sterile Dropper Bottle, Natural, Cylinder
Catalog #: UA15-415-10-BOT-15CR Quantity per Box: 100Product Features: 15 mL, Bottle, Custom Dropper, UA15-415
Customizable OptiDropperTM
Ophthalmic Dropper Bottles
Select a Bottle
Part Number
EA Per Box
Total EA
Unit Price
Price
Select a Tip or Tip/Cap
Part Number
EA Per Box
Total EA
Unit Price
Price
Select a Cap
Part Number
EA Per Box
Total EA
Unit Price
Price
Frequently Asked Questions
What final release testing is performed for these bottle products?
| Test | Method | Component |
| Sterility | Direct Transfer Method Embedded Spore Strip accredited to the ISO 17025 | Bottle, Tip, Cap |
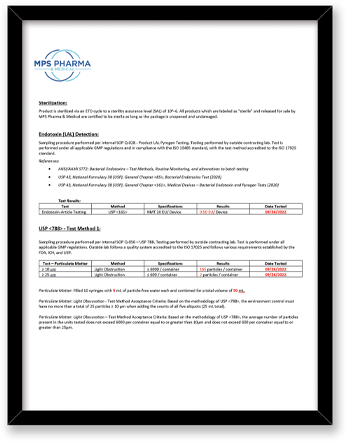
| Endotoxin/LAL | ISO 17025 – (USP), General Chapter <85>, (USP), General Chapter <161>, Medical Devices | Bottle, Tip, Cap |
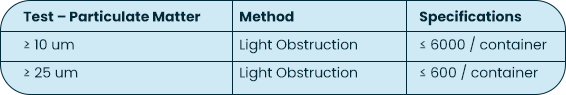
| USP <788> Test Method 1 – Particulates | Test Method Acceptance Criteria: Based on the methodology of USP <788> | Bottle, Tip (Testing limited to fluid contact surfaces) |
All testing is performed by qualified third party testing labs contracted with MPS Pharma. The final measurements are all reported on our current Certificate of Analysis.
Why do you measure particulates to USP <788>?
Our bottles are most used for topical ophthalmic preparations in the form of eye drops. This is a form of extraocular application of medication to the eyes. Specific chapters in USP guidelines state as follows:
USP <771> Ophthalmic Products – Quality Tests:
“Two general categories apply for product administration to the tissues in the eye. Intraocular administration includes all ophthalmic products that cross (penetrate) boundary tissue, such as the cornea and sclera. For subvisible content, USP guidance is followed. Products for intraocular use must comply with Particulate Matter in Ophthalmic Solutions <789>. Product for extraocular use must comply with Particulate Matter in Injections <788>.”
Both USP <788> and <789> are applicable to finished drug products; containers are not considered finished drug product. The testing MPS Pharma has performed on the bottles is based on the methodology of USP <788>, with particulate measurements reported for 10 um and 25 um sizes against limits reported on a per container basis.
Can I see a sample Certificate of Analysis that comes with this product?
See the attached PDF for a sample Certificate of Analysis
I have a special requirement (new pack size, special drop size, etc). Can you help?
We can and are willing to provide our customers options that aren’t available in our existing catalog. We are always open to adding them. Much of the depth in our catalog is specifically due to fulfilling requests just like this. Contact us with your requirements to start collaborating today.
Are the parts interchangeable? How does the numbering system work on these parts?
The interchangeable/mating parts in our catalog are designated with a special numbering system. These can be identified with the pre-fix.
All items with a UA8-425 pre-fix are compatible with each other.
All items with a UA15-415 pre-fix are compatible with each other.
UA8-425 & UA15-415 items are not compatible with each other.
If I open the pouch, will the bottle contents still be sterile?
Once the pouch has been opened, then by the definition the parts cannot be considered sterile, unless the pouch is opened in a controlled sterile environment.
What is the drop size for these bottles?
Drop size is dependent on several factors including the product being dispensed and the technique in which it is dispensed. The best advice for customers is to test the dropper tip with the compounded product to determine its suitability in its application.
A general water reference point can be provided to our customers as a starting guideline:
| Size(s) | Drop Size |
| 3 mL | 42 ul +/- 5 uL |
| 7, 10, 15, 30 mL | 40 ul +/- 5 uL |
What are the bottles made of? Can more information be shared?
The bottle and tip itself are composed of low-density polyethylene resin (LDPE). These are the fluid contact surfaces. The cap itself is composed of polypropylene (PP) and can be considered a non-fluid contact surface.
A more detailed material profile is available to share with customers who complete a non-disclosure agreement.
Are dimensional drawings of the bottle available?
Detailed dimensional drawings are available to customers.
Do you carry bottles that offer UV protection? Is there data available to share?
MPS carries a series of white bottles and tips that can be used in conjunction with colored caps to create a container closure system that protects a solution from UV light.
Specific testing standards surrounding UV protection of solutions are always performed using the container closure system itself plus the drug contents. MPS does not provide drug with the bottle, therefore no meaningful data can be provided that our end user pharmacies may leverage for their own purposes.
We can anecdotally report however that our customers do routinely use specific products of ours for light protected solutions. Those same customers do report the bottle, in combination with their drug product, pass the UV protection requirement as defined in the existing testing standards.
Please reach out to us to find out which items would work best in supporting a successful passage of these tests.
Can I get a specification sheet of this item for my records?
See the attached document for the specification sheet
Our pharmacy follows USP <797> and it requires we use depyrogenated materials. Are your products depyrogenated? Do you have a Certificate of Analysis (C of A) stating such?
All MPS lots are tested for endotoxins (pyrogens) and will meet the materials requirements as defined in USP <797>. A detailed explanation of this can be found in this PDF link. All MPS C of As include measurements for these endotoxins.
Have any questions?
Contact our Customer Service team for additional support at
(844) 641-3814 or by email at sales@mpspharma-inc.com

 0
0