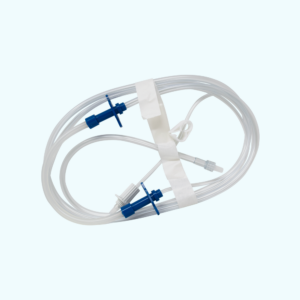
Baxter REPEATER™ Pump Tube Set (H93863 equivalent) with Three Universal Spikes, Luer Lock, External Thread
Manufacturer-Direct, 100% Baxter Compatible, Triple Lead High Volume Pump Chamber Set, Length 125? Macrobore Tubing, Large 0.2 Micron Vented Air Spike, Pinch Clamp, Silicone Boot, Male Luer Lock, Sterile
Not the set you are looking for? See our complete line of 100% Baxter Compatible Pump Tube Sets.
Reliable, high quality pump tubing. Item is compatible with all models of the Baxter REPEATER™ Compounding Pump.
Pump tubing, in use with pump, can be used for various pharmacy applications including:
- Drug vial reconstitution
- Admixture preparation
- Sterile syringe filling
- Ambulatory pump filling
- Oral dispenser filling
- Diluent transfers of small to large volumes
Product carries a male luer lock connector along with contains three (3) bag spikes compatible with most pharmacy applications. Product allows a user to connect to multiple source bags at once.
Product ships sterile, endotoxin limit tested and comes with 10 EA per box.
What final release testing is performed for these products?
| Test | Method |
| Sterility | Direct Transfer Method Embedded Spore Strip accredited to the ISO 17025 |
| Endotoxin/LAL | ISO 17025 – (USP), General Chapter <85>, (USP), General Chapter <161>, Medical Devices |
All testing is performed by qualified third party testing labs contracted with MPS Pharma. The final measurements are all reported on our current Certificate of Analysis.
Can I see a sample Certificate of Analysis that comes with this product?
See the attached PDF for our current Certificate of Analysis
Can I get a specification sheet of this item for my records?
See the attached document for the specification sheet
Do you have a comparative document of your set versus the original Baxter Brand?
See the attached document for comparison
Our pharmacy follows USP <797> and it requires we use depyrogenated materials. Are your products depyrogenated? Do you have a Certificate of Analysis (C of A) stating such?
All MPS lots are tested for endotoxins (pyrogens) and will meet the materials requirements as defined in USP <797>. A detailed explanation of this can be found in this PDF link. All MPS C of As include measurements for these endotoxins.

 0
0